Introduction
Alzheimer’s disease is one of the most common neurodegenerative disorders in the elderly. The chance to get Alzheimer drastically increases in the elderly group; from a measly 0.26% in the middle age group to a massive 26% for individuals older than 851. Given the nature of the disorder, it is more of a long-term development of symptoms.
Polyphasic sleeping with sleep reduction mechanics definitely can cast doubts on its long-term potential. While this is a legitimate concern, there has been no official publication on polyphasic sleepers getting Alzheimer. A lot of topics need more research for conclusions; however, as always we have a few words of cautions for aspiring polyphasic sleepers.
Sleep-wake Patterns in Alzheimer’s Patients
Altered sleep patterns are a common sign with people suffering from Alzheimer’s disease. In addition, it is somewhat unclear if Alzheimer’s or altered sleep patterns are the trigger. However, some research only speculates which part is the trigger. Meanwhile, other papers might give a hint which one is the trigger and which one is the result of said trigger.
Thus, considering all aspects, the relation between sleep and Alzheimer’s is still under research. Scientists are working hard to uncover its full relationship with sleep.
- Regardless, Alzheimer’s patients usually have more sleep disturbances and changes in their sleep-wake rhythm1,2.
- Their sleep quality is shallow, and they spend more time “awake” when they’re supposed to be sleeping. As a result, they spend more time in light sleep and receive much less restorative sleep stages than healthy individuals1. This does not, however, mean that a change in your rhythm would develop Alzheimer’s.
- Disordered sleep or bad sleep that results in sleep deprivation of some kind has, however, been a cause for Alzheimer’s3,4. Thus, this strengthens the point that people should have a reasonably consistent sleep pattern and minimize sleep deprivation periods.
Vital Sleep in Alzheimer’s Patients
- As one article points out, SWS reduction is especially connected to the development of Alzheimer’s. This is a probable cause because of the fact that the glymphatic system operates at its highest capacity during SWS. In addition, it is responsible for clearing out metabolic waste5.
- A small percentage of REM sleep compared to the total amount of sleep is also possibly a culprit for developing Alzheimer’s. However, polyphasic sleepers often have a much higher percentage of REM sleep compared to monophasic sleepers.
- Most notably, the total duration REM usually remains intact in stable adaptations. It is, therefore, uncertain how the adaptation process affects the development of Alzheimer’s.
- The safe assumption is still that REM deprivation is a risk regarding the development of Alzheimer’s.
Polyphasic Schedules & Their Safety for Alzheimer’s Development
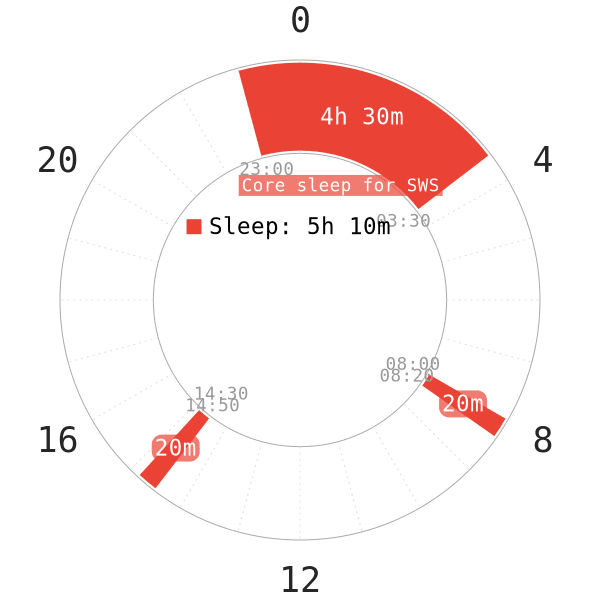
From the aforementioned findings, nap-only schedules carry a risk. This is because they reduce the duration of SWS and REM in regular sleepers. A healthy polyphasic schedule should not bring about Alzheimer’s as long as one adapts successfully.
- Furthermore, a healthy polyphasic schedule should have a reasonably long core sleep to cover all personal SWS requirements.
- The naps, on the other hand, will support the remaining REM sleep.
It is also important to maintain a healthy circadian rhythm; circadian disruptions has a direct link with developing Alzheimer’s6. Elevated beta amyloid levels, which are the waste products of the brain responsible for Alzheimer’s, reduce human’s cognitive performance7. Refer to the polyphasic schedule outline below for assessing Alzheimer’s risks.
The community is currently developing a testing application to better examine the effects of pre-, during and post-adaptation polyphasic sleep on cognitive performance.
Holistic Assessment of Polyphasic Schedules Against Alzheimer
NOTE:
- The table does not account for short sleepers. Instead, it only considers average sleepers with normal sleep architecture.
- The normal sleep baseline is 90m SWS and 90m REM daily as a representation.
- Assessment denotation: Green = safe, yellow = middling risk, red = high risk
| Total sleep time (hours) | Safety Ranking |
| At least 7.5h | |
| 6h or more | |
| No less than 5h | |
| No less than 4h | |
| Below 4h but above 3h | |
| No less than 2h | |
| Less than 2h |
Table 1. Total Sleep Time on Polyphasic Schedules and their safety against Alzheimer’s
Brief Analysis
- Most schedules with at least 4h TST should be safe against Alzheimer’s.
- If your SWS requirements are high (e.g, 2.5h daily), a 4h TST schedule won’t cover all your necessary SWS and REM requirements. Thus, consider the minimum sleep threshold from your vital sleep carefully!
- Even though a 4h TST schedule seems safe, if you have no concrete core sleeps and only naps (e.g, 60m naps as longest sleep duration), you are also exposing yourself to higher risks for Alzheimer. Aside from a much harder adaptation, you likely will constantly interrupt your SWS cycle.
- Friendly reminder: There are current polyphasic schedules with below 4h total sleep that are not nap-only. For example, Everyman 4 and Everyman 5. Each of these also has a core sleep.
- All of these warnings, however, do not account for short-term nap-only practice. Buckminster Fuller, for example, practiced Dymaxion for approximately 2 years; yet, he never developed Alzheimer later on8.
Main authors: Crimson & GeneralNguyen
Last page update: 15 February 2021
Reference
- MOE, K. E., VITIELLO, M. V., LARSEN, L. H., & PRINZ, P. N. (1995). Sleep/wake patterns In Alzheimer’s disease: relationships with cognition and function. Journal of Sleep Research, 4(1), 15–20. doi:10.1111/j.1365-2869.1995.tb00145.x.
- Binkhorst R, Pool J, van L, Bouhuys A. Maximum oxygen uptake in healthy nonathletic males. Int Z Angew Physiol. 1966;22(1):10-18. [PubMed]
- Carter N, Shiels A, Jeffery S, et al. Hormonal control of carbonic anhydrase III. Ann N Y Acad Sci. 1984;429:287-301. [PubMed]
- Aguayo A, Nair C, Midgley R. Experimental progressive compression neuropathy in the rabbit. Histologic and electrophysiologic studies. Arch Neurol. 1971;24(4):358-364. [PubMed]
- Tarasoff-Conway J, Carare R, Osorio R, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457-470. [PubMed]
- Dutt M. Preparation of Schiff-type dye-SO2 reagents with phosphoric acid and their cytochemical properties. Microsc Acta. 1981;84(2):143-146. [PubMed]
- Adenis J, Serra F. Bipalpebral sliding flap in the repair of inner or outer canthal defects. Br J Ophthalmol. 1986;70(2):135-137. [PubMed]
- Wikipedia Contributors. “Buckminster Fuller.” Wikipedia, Wikimedia Foundation, 24 Mar. 2019, en.wikipedia.org/wiki/Buckminster_Fuller.